FDA's Tablet 'Scoring' Guidance Aims to Bridge Generic, Brand Divide


Safer eyedrops will require new FDA powers, experts say
Dr. Reddy's Can't Sell Generic Opioid Treatment, Judge Rules - Bloomberg
Amid flurry of new cancer drugs, how many offer real benefits?
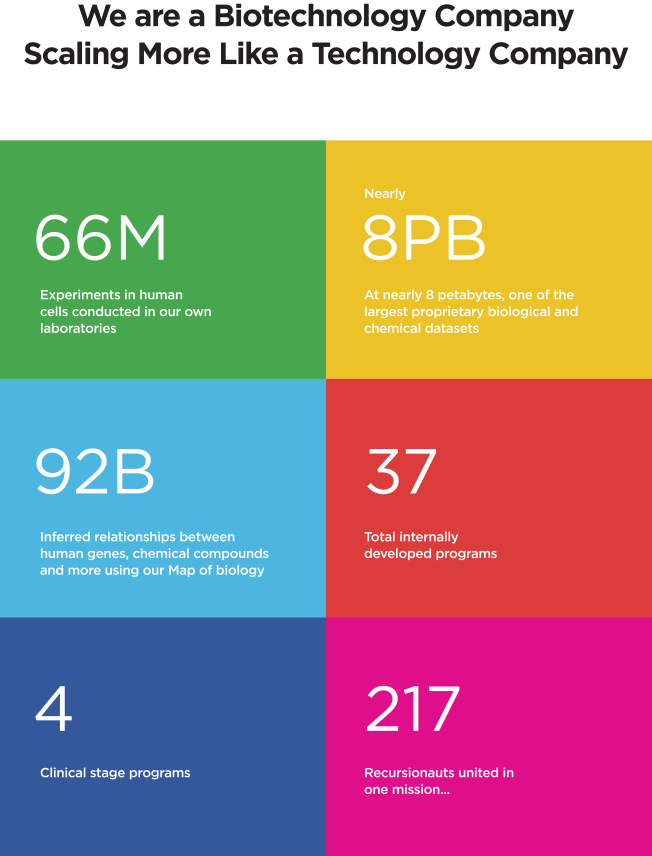
424B4
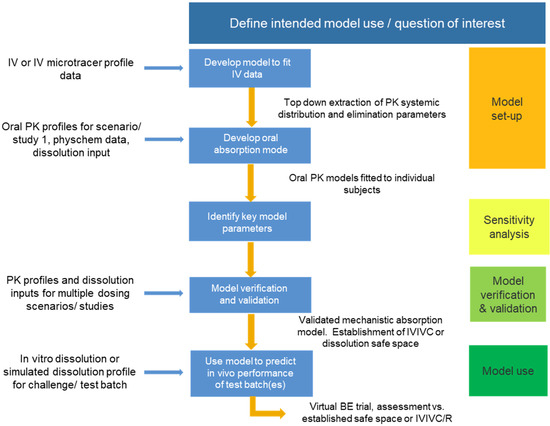
Pharmaceutics, Free Full-Text

Amitriptyline Hydrochloride Tablets, USPRx only

READ: Federal ruling to block FDA approval of abortion pill

Improved Early Detection of Drug-Induced Liver Injury by Integrating Predicted in vivo and in vitro Data

Changing Strategy Between Bridge to Transplant and Destination LVAD Therapy After the First 3 Months: Analysis of the STS-INTERMACS Database - Journal of Cardiac Failure

Deep learning in image-based phenotypic drug discovery: Trends in Cell Biology

Guidance For Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation with Regulatory Requirement for Other Regulated Market.

Guidance For Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation with Regulatory Requirement for Other Regulated Market.

Seven Noteworthy 505(b)(2) NDA Submissions









