Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Proficiency Testing Regulations Related to Analytes and Acceptable Performance


CLIA-Certified CAP-Accredited Lab
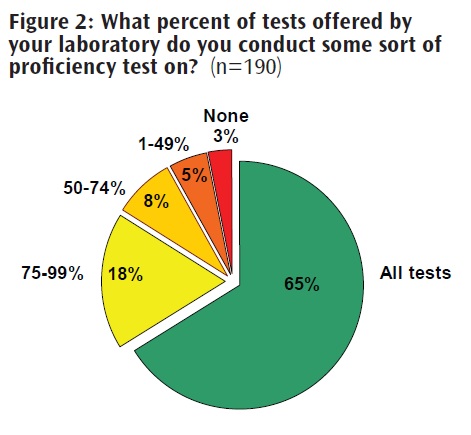
Public Health at Risk The Pew Charitable Trusts

CLIA Regulations Changing in 2024

PROFICIENCY TESTING. Clinical Laboratory Improvement Amendments (CLIA) DOs and DON Ts. Brochure # 8 - PDF Free Download
CLIA Regulations Changing in 2024

Representation Under CLIA - National Society for Histotechnology

Clinical Laboratory Improvement Amendments of 1988 (CLIA) Proficiency Testing Regulations - CMS-3355-F

license & certification — allergenis provider site
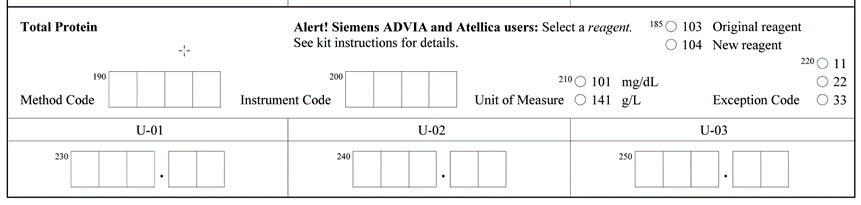
Proficiency Testing/External… College of American Pathologists

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories
PDF) CLIA requirements for proficiency testing: the basics for laboratory professionals

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories